J.T.Baker® brand

For the world's most demanding applications
Biopharmaceutical and semiconductor manufacturers, as well as environmental testing laboratories, are just a few of the customers who trust the application-optimized and function-tested products from the Avantor J.T.Baker® brand to facilitate reliable results that maximize productivity and efficiency.
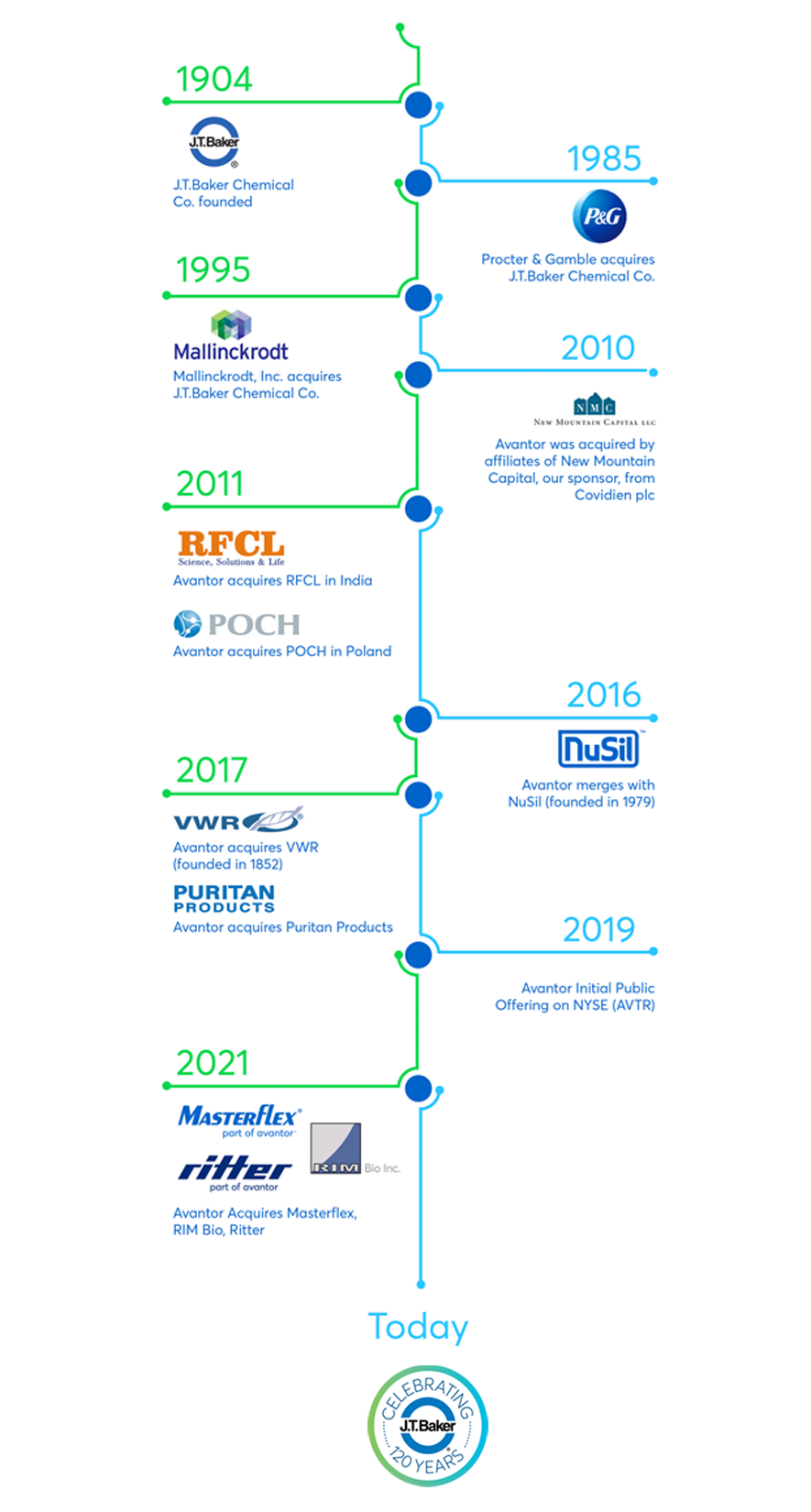
A legacy of excellence since 1904
For more than a century, the J.T.Baker® brand has stood for excellence from the laboratory to full-scale production.
In 1904, John Townsend Baker founded the J.T.Baker Chemical Company with a mission to produce chemicals of the "highest degree of purity commercially available." As the first to include lab results listing trace impurity levels on labels, J.T.Baker® chemistries quickly established a reputation for quality, data transparency and collaboration.
The quality you want for the results you need
Today, the J.T.Baker® brand is one of the most widely known and respected brands of chemicals around the globe. They are backed by Avantor's global quality and supply chain systems, cGMP manufacturing capabilities and change notification initiatives to ensure the products are of consistently high-performance and high-purity.
J.T.Baker® brand products help:
- Reduce rework and out-of-spec products
- Achieve higher manufacturing efficiencies
- Maximize the value of high-tech equipment
- Achieve faster pharmaceutical regulatory approval
Product overview
Choose from a portfolio of products that support a variety of applications and manufacturing processes, including:
- Analytical chemistries for Ultra LC/MS, LC/MS, HPLC and GC
- Sample preparation products including SPE columns and disks.
- High-purity salts, solvents and reagents
- High-purity acids for elemental impurity analysis
- Biological buffers and reagents High Purity Low Endotoxin (HPLE) sugars
- Process Chromatography Media
- Innovative packaging options, including J.T.Baker® Direct Dispense packaging
- Salts and solutions
Reduce rework and out-of-spec products

Achieve higher manufacturing efficiencies

Maximize the value of high-tech equipment
Achieve faster pharmaceutical regulatory approval
 J.T.Baker® products
J.T.Baker® products Literature
Literature Video
Video Contact us
Contact us Participate & Win
Participate & Win